JATC1
Work Packages
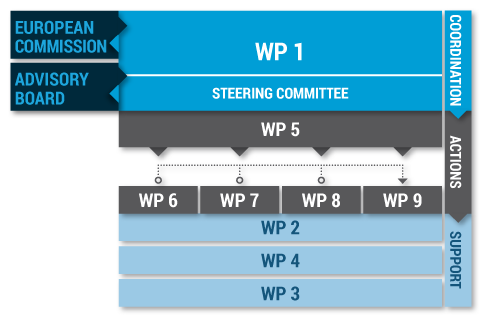
The JATC1 is a 36-month project that consists of an integrated sequence of nine (9) work packages (4 horizontal and 5 core WPs).
WP1
Leader
HCS
GREECE
Coordination
Hellenic Cancer Society
Aim: To manage the JATC1 procedures and to make sure that it is implemented as planned
WP2
Leader
BATUT–IPHS
SERBIA
Dissemination
WP participants: HCS, MoH CY, SE
Aim: To maximize the JATC1 impact and ensure that the project’s outcome will be made available to the target groups
WP3
Leader
AGES
AUSTRIA
Evaluation
WP participants: HCS
Aim: To create and implement an evaluation plan that will describe the criteria and methods for project evaluation, as well as the procedures and tools for project‘s quality assurance
WP4
Leader
MoH CY
CYPRUS
Integration into National Policies and Sustainability
WP participants: HCS, NCPHA, TA, ANSES, BfR, SE, HSE, IRCCS-IRFMN, NTAKD, CSJA
Aim: To enhance effective and efficient integration into national policies and training opportunities and ensure longer term sustainability of the JATC1
WP5
Leader
SIK
DENMARK
EU Common Entry Gate (EU-CEG) data Extraction and Handling
WP participants: HCS, AGES, BHTC, NCPHA, TA, ANSES, MoH IT, NTAKD, NOMA, MS-DGS, NLZOH, CSJA, UK-DH
Aim: To provide the framework for the efficient usage of the data submitted in the EU-CEG
This WP is the key WP for providing access to data
WP6
Leader
ICO
SPAIN
Tobacco Product Evaluation
WP participants: HCS, AGES, BHTC, SIK, ANSES, BfR, APTL-CERTH, HSE, IRCCS – IRFMN, ISS, MoH IT, NTAKD, RIVM, NIPH, MS – DGS, NZLOH, CSJA, UK-DH, HTS
Aim: To handle aspects pertinent to tobacco product data handling collected by EU MS, in light of the obligations of the TPD
WP7
Leader
HCS
GREECE
E-cigarette Product Evaluation
WP participants: AGES, BHTC, ANSES, BfR, APTL-CERTH, DOHI, HSE, IRCCS-IRFMN, ISS, MoH IT, HI, NTAKD, RIVM, NIPH, NOMA, MS-DGS, ICO, CSJA, UK-DH, HTS
Aim: To support the EU MS activities in ensuring that electronic cigarettes and refill containers are only placed on the market if they comply with the TPD
WP8
Leader
IRCCS-IRFMN
ITALY
Laboratory Verification, Collaboration and Analyses
WP participants: HCS, AGES, BfR, APTL-CERTH, NVSPL, RIVM, NLZOH, CSJA, HTS
Aim: To develop collaborations across EU MS independent laboratories and identify suggested methodologies and test analyses for reporting homogenization
WP9
Leader
RIVM
THE NETHERLANDS
Additives Subject to Enhanced Reporting Obligations
WP participants: HCS, SIK, ANSES, BfR, IRCCS-IRFMN, ISS, NIPH, CSJA
Aim: To aid EU MS in the evaluation of data submitted with regards to enhanced reporting obligations under TPD Art. 6 which shall apply to certain additives contained in cigarettes and roll-your-own tobacco that are included in a priority list outlined in the Commission Implementing Decision 2016/787